The Rizzo Laboratory for Translational Glycobiology is part of the Acute Lung Injury and Infection Center of Excellence at the University of Pittsburgh School of Medicine. Our overarching goal is to develop glycan-targeted precision medicine approaches to treat critical illness syndromes including ARDS, pneumonia, and sepsis. To accomplish this we have three primary areas of investigation:
1. Mechanistic Investigation
Mechanisms of epithelial glycocalyx support of pulmonary surfactant
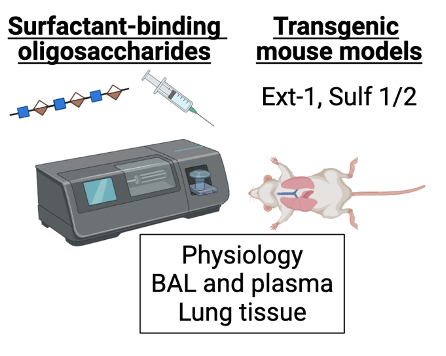 Acute respiratory distress syndrome (ARDS) is a common cause of respiratory failure in the ICU that is associated with a 30-40% mortality rate. A key hallmark of this disease is dysfunction of pulmonary surfactant, a substance that is composed of phospholipids and proteins that serves to prevent collapse of the alveoli during the normal breaths. However, despite decades of research, the mechanisms underlying surfactant dysfunction in ARDS are incompletely understood. Our recent research demonstrates that the alveolar epithelial glycocalyx, a layer of glycosaminoglycans that coats the apical surface of alveolar epithelial cells, is damaged in multiple different mouse models of ARDS and that targeted degradation of this structure alone decreases lung compliance by impairing surfactant function. Additionally, we discovered that in patients with ARDS, airspace glycosaminoglycan (GAG) shedding (a measure of glycocalyx degradation) correlates with the degree of hypoxemia and predicts the duration of mechanical ventilation (Rizzo et al. JCI Insight 2022), underscoring the clinical relevance of this work. We are currently expanding on this work to further define the molecular mechanisms by which the epithelial glycocalyx supports surfactant dysfunction, which is necessary in order to understand the pathogenesis of ARDS and develop novel treatments to improve lung function and outcomes in our ICU patients. This work includes sophisticated in vitro assays and lung injury models using transgenic mouse models with alterations in the biosynthesis of heparan sulfate, a key component of the glycocalyx.
Acute respiratory distress syndrome (ARDS) is a common cause of respiratory failure in the ICU that is associated with a 30-40% mortality rate. A key hallmark of this disease is dysfunction of pulmonary surfactant, a substance that is composed of phospholipids and proteins that serves to prevent collapse of the alveoli during the normal breaths. However, despite decades of research, the mechanisms underlying surfactant dysfunction in ARDS are incompletely understood. Our recent research demonstrates that the alveolar epithelial glycocalyx, a layer of glycosaminoglycans that coats the apical surface of alveolar epithelial cells, is damaged in multiple different mouse models of ARDS and that targeted degradation of this structure alone decreases lung compliance by impairing surfactant function. Additionally, we discovered that in patients with ARDS, airspace glycosaminoglycan (GAG) shedding (a measure of glycocalyx degradation) correlates with the degree of hypoxemia and predicts the duration of mechanical ventilation (Rizzo et al. JCI Insight 2022), underscoring the clinical relevance of this work. We are currently expanding on this work to further define the molecular mechanisms by which the epithelial glycocalyx supports surfactant dysfunction, which is necessary in order to understand the pathogenesis of ARDS and develop novel treatments to improve lung function and outcomes in our ICU patients. This work includes sophisticated in vitro assays and lung injury models using transgenic mouse models with alterations in the biosynthesis of heparan sulfate, a key component of the glycocalyx.
This work is funded by the Parker B Francis Foundation.
2. Translational Human Subjects Investigation
Impact of glycocalyx degradation on ARDS physiology and outcomes
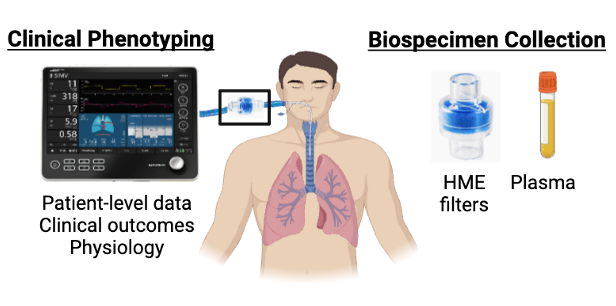
Acute respiratory distress syndrome (ARDS) affects over 200,000 US patients per year and has a 30-40% mortality rate. Numerous attempts to develop effective therapies for ARDS have failed, which is increasingly attributed to substantial patient-level mechanistic heterogeneity. This heterogeneity has inspired efforts to identify “treatable traits” in ARDS: causal mechanisms that can be identified and targeted to produce meaningful benefit to patients. The pulmonary endothelial and epithelial glycocalyces are glycosaminoglycan-enriched layers that line the lung vasculature and airspaces respectively. Glycocalyx degradation contributes to lung injury in mice, and biomarkers of glycocalyx degradation are associated with worse outcomes in ARDS patients. These data suggest that glycocalyx degradation may be a treatable trait in ARDS. This project will estimate the attributable impact of glycocalyx degradation on ARDS outcomes and determine the longitudinal relationship between glycocalyx degradation and the dynamic physiology of ARDS, thereby establishing a framework to translate these mechanistic discoveries into effective therapies. We are partnering with the University of Pittsburgh Acute Lung Injury Registry to conduct a prospective cohort study, which includes patient-level data, longitudinal bedside physiologic assessments, and serial biospecimen collection (airspace fluid and plasma). We will employ causal inference approaches to estimate the attributable impact of glycocalyx degradation on patient outcomes and longitudinal modeling to determine the relationship between glycocalyx degradation and the dynamic physiology of ARDS. This work will elucidate the patient-specific and injury-specific factors driving glycocalyx degradation. It will also determine timing of glycocalyx degradation during critical illness and quantify its impacts on physiology and outcomes, which is necessary for the implementation of precision medicine approaches.
3. Development of rapid diagnostics
Optimization of a rapid assay to quantify circulating glycosaminoglycans in plasma
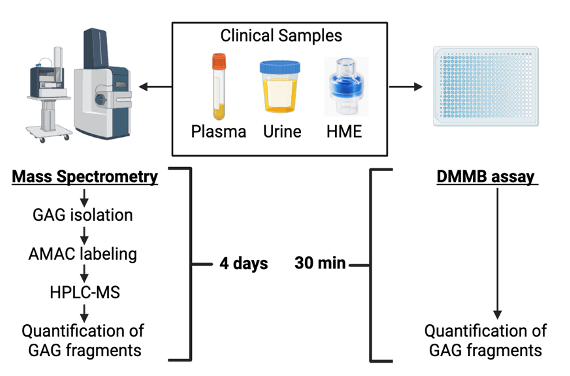
Endothelial glycocalyx degradation is a driver of sepsis pathogenesis and contributes to worse outcomes. Although mass spectrometry is the gold standard approach for measuring glycocalyx fragments in clinical samples, it is ill-suited for bedside use due to its high expense, long processing time, and the degree of technical expertise required for these analyses. To address the need for a rapid, inexpensive, point- of-care assay of soluble GAGs, we developed the dimethylmethylene blue (DMMB) assay, a colorimetric assay of sulfated GAGs that can be performed easily in unprocessed human urine and airspace fluid. However, the accuracy of DMMB in plasma is unknown. Our current work seeks to optimize DMMB as a measure of plasma GAGs. Alternatively, urine DMMB could serve as a proxy for plasma DMMB and we will explore whether urine DMMB could be a proxy of GAG shedding. This work is critical to our long-term goal of developing and implementing glycan-targeted precision medicine approaches in critical illness, as it will enable rapid identification of the subgroup of patients who are most likely to derive benefit from therapies aimed at enhancing glycocalyx function.
This work is done in collaboration with the laboratory of Dr. Eric Schmidt at Massachusetts General Hospital and is funded by an NIGMS R21 award (Schmidt/Shapiro).